Authors - Moataz O Abu-Al-Saud, Soheil Esmaeilzadeh, Amir Riaz, Hamdi A Tchelepi
Download the Conference Version
Abstract - Understanding the effect of injected water salinity is becoming crucial, as it has been shown to have a strong impact on the efficiency of oil recovery process. Various experiments have concluded that carbonate wettability is altered when the water ionic-composition is changed. In this work, a numerical investigation of an oil blob mobilized by water is conducted inside a single pore. The presented model studies the synergy effect of multiphase flow and water salinity at the pore level.
To model the multiphase flow at the pore-scale, the full hydrodynamic Navier-Stokes equations are solved using direct numerical simulation (DNS). The effect of brine ionic-composition is examined through the electric double layer effect. Experimental zeta potential values, published in the literature, of crude oil/water and water/carbonate interfaces have been employed in the model, which capture the water salinity effect.
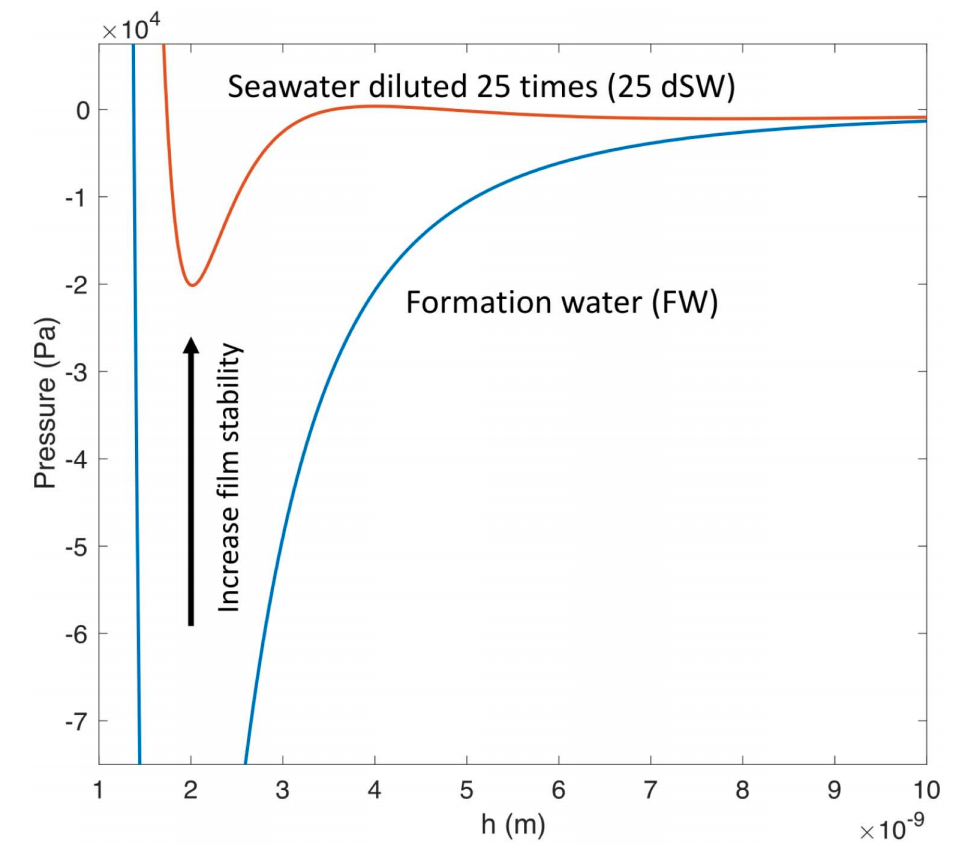
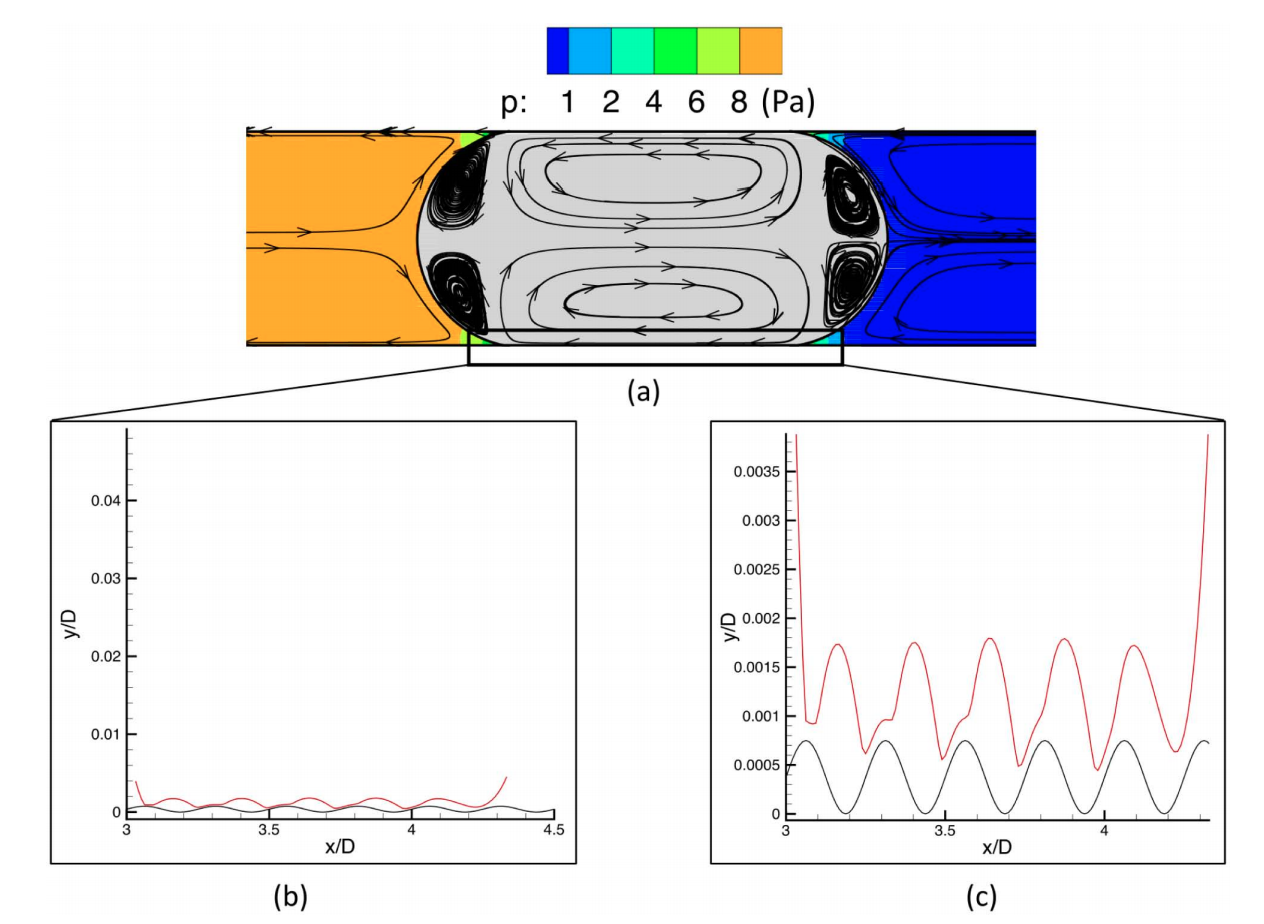
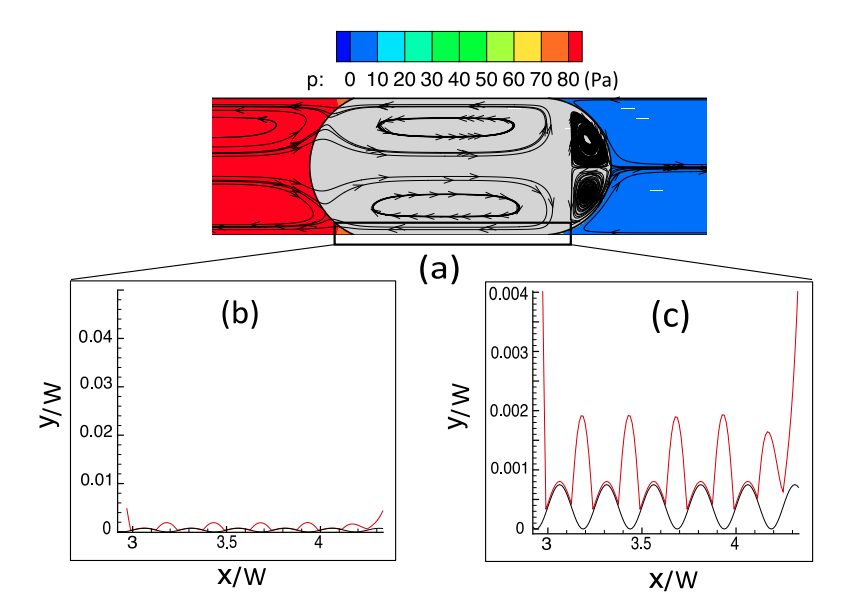
The simulation results show that the water wetting film surrounding the oil-droplet collapses to an adsorbed nanometer water layer when high salinity water is used. As a result, a large pressure gradient is required to mobilize the oil inside the pore and overcome the attractive surface forces between the oil/water and water/carbonate interfaces. For low-salinity injected water, the carbonate surface becomes more waterwet. The wetting film surrounding the oil blob becomes stable due to the repulsive electric double layer force. Therefore, less energy is required to mobilize the oil blob inside the pore compared to high water salinity. The effect of solid roughness and injected water flow rate are also studied, which show to have a strong impact on the oil displacement efficiency.
The novelty of the numerical method lies in efficiently capturing the nanoscale effect of the electric double layer in pore-scale multiphase flow at the microscale. The simulation results provide fundamental insights on the efficiency of low-salinity waterflooding at the pore level.
Keywords: Two-phase flow, Porous media, Wettability alteration, Ionic composition, Thin-films
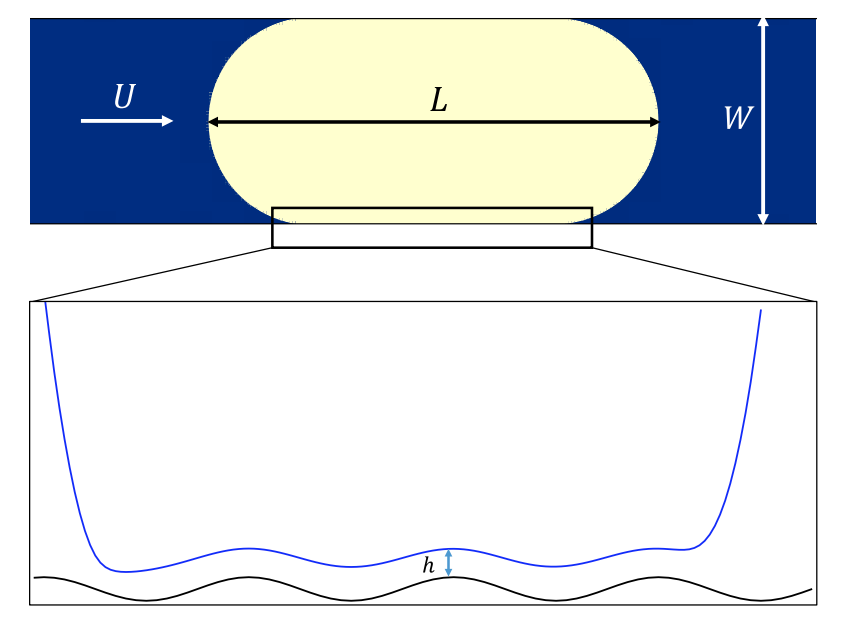
Fig.1. Schematic of a moving droplet inside a micro-channel in the presence of a solid wall with low amplitude roughness. $h$ is the thickness of the thin-film deposited on the solid surface surrounding the moving droplet. $L$ is the corresponding length of the moving droplet, $U$ is the velocity corresponding to the injection flow rate imposed at the inlet (left side) of the channel, and $W$ is the channel width).